 Research Article
Research Article
Effectiveness Of the Antispasmodic Pridinol Vs. Nsaids in Patients with Acute (Low) Back Pain – Results of Providence, A Retrospective, Non-Interventional Propensity- Score Matched Dual Cohort Analysis of Depersonalized 4-Week Real-World Data Provided by The German Pain E-Registry
Michael A Überall1*, Artur Schikowski2, Johannes Horlemann3 and Gerhard HH Müller-Schwefe4
1Institute of Neurological Sciences - IFNAP, Center of Excellence in Health Care, Research, Nordostpark 51, 90411 Nürnberg, Germany
2Pain Institute & Center for Medical Education Düsseldorf, Friedrichstraße 13-15, 40217 Düsseldorf, Germany
3German Pain Association, Lennéstraße 9, 10785 Berlin, Germany
4Interdisciplinary Pain and Palliative Care Center Göppingen, Schillerplatz 8/1, 73033 Göppingen, Germany
Michael A Überall, Institute of Neurological Sciences - IFNAP, Center of Excellence in Health Care, Research, Nordostpark 51, 90411 Nürnberg, Germany
Received Date:February 27, 2024; Published Date:March 12, 2024
Abstract
Background: Current guidelines recommend the preferred use of NSAIDs for the treatment of acute (low) back pain [a(L)BP] for a limited
period of time, although their effects in controlled clinical trials is at best manageable and effect sizes of questionable clinical relevance. Positive
recommendations for antispasmodics (such as pridinol, PRI) are frequently lacking, with reference to allegedly insufficient study data and generalized
statements about “unfavorable” side effect profiles. Practical comparisons of both therapy alternatives under daily-life conditions are lacking.
Methods: Retrospective evaluation of depersonalized 4-week data from the German Pain e-Registry on two cohorts of patients with a(L)BP in
whom complaints weren´t sufficiently alleviated either spontaneously or by self-treatment with OTC and who were then 1st line prescribed either
NSAIDs or PRI as monotherapy. Propensity score-based data selection to ensure comparable baseline situations (n=467 per cohort). Responder
categorization for the primary endpoint based on a composite of (a) absence of treatment discontinuation due to drug-related adverse events
(DRAEs), (b) evidence of significant and clinically relevant improvements of pain intensity, (c) pain-related functional impairments in daily life, (d)
physical and (e) mental quality of life.
Results: Confirmatory evidence of a significant superior response rate with PRI vs. NSAIDs: 69.0% (66.0-71.9) vs. 34.0% [31.0-37.1; p<0.001,
effect size 0.349; OR: 4.3 (3.3-5.7), RR: 2.1 (1.8-2.4); NNT: 2.9], including greater improvements with PRI vs. NSAIDs in all pain and functional
parameters evaluated vs. BL (mean±SD): pain intensity (PIX): 73.0±16.4 vs. 62.8±22.8%, functional restrictions (mPDI): 69.0±16.6 vs. 42.0±30.2%,
physical QoL: 29.7±10.3 vs. 18.2±8.2%, mental QoL: 18.5±11.1 vs. 13.5±11.7% (p<0.001 for each). Significantly less DRAEs (9.0 vs. 20.8%; p<0.001,
ES: 0.165; OR: 0.377, RR: 0.565; NNH: 8.5) and DRAE-related treatment discontinuations (3.4 vs. 9.4%; p<0.001, ES: 0.122; OR: 0.341, RR: 0.517;
NNH: 16.7) among PRI vs. NSAIDs.
Conclusions: Based on the present retrospective analysis of non-interventional collected health care data of patients with a(L)BP, the first-line
prescription of the antispasmodic PRI over 4-weeks was found to be superior effective compared to the guideline-compliant prescription of NSAIDs
when spontaneous remission was inadequate and self-treatment with OTC NSAIDs/analgesics was ineffective.
Keywords:Pridinol; Antispasmodic; Nsaid; Acute (Low) Back Pain; Short-Term Treatment; Propensity Score Matching
Introduction / Background
Acute (low) back pain [a(L)BP] is one of the most common reasons for adults to see a general practitioner or to visit an emergency department [1,2] and with nearly 80-90% the majority of adults in industrialized countries of the western world will experience a(L)BP at least once in their lifetime [3]. Although a(L) BP usually resolves spontaneously in many patients affected or recovers substantially within few weeks following self-medication, two-thirds of patients report either persistent pain or functional disability at three months. After recovering from an initial a(LBP) episode, one-third of affected patients experiences recurrent episodes within the next 12 months which are associated with a statistically significant increased risk of developing chronic (L) BP (c(L)BP) [4-7], the latter turning into the world’s most leading cause of disability, predicted to increase even further in the coming decades with respect to sick leave, lost working days, early retirement and years lived with disability [8].
The aim of any physician driven a(L)BP treatment is to increase rate and extent of spontaneous pain relief, speed up recovery from functional disability and to minimize the risk of recurrent events or the progression into a chronic pain syndrome [9]. Beyond the usual recommendations regarding counselling, reassurance and physical activity, most guidelines recommend the use of nonsteroidal antiinflammatory agents (NSAIDs) as first line medical measure in patients with a(L)BP [10], despite the fact that a current Cochrane analysis revealed that the available evidence for NSAIDs in shortterm reduction of a(L)BP intensity is only of moderate quality and effect sizes (ES) reported only of subclinical relevance. vs. placebo [11]. Furthermore, a recently published translational study of animal data and data from a UK-based patient registry demonstrated that initial immunosuppressive a(L)BP treatment mediates the transition into a chronic pain stage and that a(L)BP patients who took anti- inflammatory medications such as NSAIDs had a higher risk of ending up with persistent, chronic pain, which ultimately contradicts the medical rationale for early pharmacotherapeutic intervention [12].
In everyday practice, it is important to adapt appropriate pharmacological measures of treatment to the individual patient’s needs and requirements of the specific case and to tailor therapies according to their foreseeable effect and tolerability. In this context, the analgesic effects of any countermeasures against a(L) BP rely primarily on the mode-of-action and vary with respect to the underlying pathophysiology, while safety and tolerability depend also on the individual patient profile, which-in view of heterogeneous patient populations, the limited number of direct comparative trials, as well as the known safety and tolerability risks of NSAIDs and the latest signals on their chronification promoting effects-make it difficult to generalize recommendations of one or the other pharmacotherapeutic option as first line approach when self-medication has failed and spontaneous remission has not occurred.
In the vast majority of cases, so-called non-specific (L)BP is either caused by muscular dysfunctions or is accompanied by these in a reactive manner and leads clinically to muscle pain in the area of the spine or the adjacent regions, local tenderness, malposition, and painful movement restrictions, with patients affected showing diffuse hyperactivity and abnormal activation of the affected muscle groups in the electromyogram (EMG) [13]. If these clinical phenomena can be identified in a patient with a(L)BP, it does not appear to make much medical sense to treat the complaints with an NSAID-despite the above-mentioned guideline recommendations. Instead, an attempt should be made to select a pharmacological treatment strategy that meets the actual treatment goal (physical mobilization through rapid pain relief and alleviation of painrelated functional restrictions of daily life activities) while at the same time being safe and well tolerated. From an epidemiological and pathophysiological point of view, first and foremost muscle tone-reducing agents should be considered for patients with a(L) BP, but the available scientific evidence for agents in this substance group is limited and the medical evaluation generally suffers the problem that these agents-although they differ considerably in terms of their mode-of-action, efficacy and tolerability profile-are usually evaluated in aggregate form and hardly any meaningful clinical studies are available for the various agents nor will they be generated in the foreseeable future (in view of their advanced age and the resulting lack of patent protection). Due to recent efficacy, tolerability, and safety evaluations by the European Medicines Agency (EMA), the areas of application of the currently available active substances with a muscle tone modifying effect have been increasingly restricted in recent years. Currently, only two nonbenzodiazepine antispasmodics are approved for (L)BP and available for prescription in Germany, one of which (pridinol, PRI) was newly authorized in 2018 as a result of a bioequivalence proof compared to a reference compound available in Italy since 1961 and has since enjoyed great popularity. As for all muscle relaxants, the medical-scientific basis for the practical use of PRI is manageable. In 2022, the data of two placebo-controlled 3-week studies in 342 patients with various muscular pain syndromes were published, in which PRI showed significantly greater relief of pain intensity as well as various pain-related limitations with largely comparable tolerability vs. placebo [14]. In the same year, the results of a retrospective analysis of non-controlled data from the German Pain e-Registry (GPeR) on the use of pridinol for the relief of (L)BP under daily practice conditions has been published, which characterized PRI as a well-tolerated non-benzodiazepine antispasmodic when the recommendations for use in the product information are followed [15]. Further information on the clinical benefit-especially in direct comparison to NSAIDs – has been lacking so far, which is why the German Pain Association commissioned this study entitled PROVIDENCE to evaluate the effectiveness of PRI in comparison to NSAIDs in a(L)BP patients.
Study Objective
This study aimed to evaluate real-world clinical practice data in order to assess the 4-week effectiveness, safety, and tolerability of the nonbenzodiazepine antispasmodic PRI compared to NSAIDs as first line prescriptions in primary care patients with a(L)BP in whom physical activity and self-medication with over the counter (OTC) medication failed.
Ethics
All research reported in this publication has been conducted in an ethical and responsible manner and in full compliance with all relevant codes of experimentation and legislation. This study based on a non-interventional, retrospective evaluation of depersonalized real-world data documented via the technical structures and standards of the German Pain e-Registry (GPeR) a nation-wide, webbased pain treatment registry-before and during routine treatment of pain patients according to the legal requirements of the German quality assurance agreement for specialized pain therapy and did not require approval by an Ethics Committee. This study followed the principles of the Declaration of Helsinki, and the study protocol has been reviewed and approved by the executive boards of the German Pain Association and the German Pain League prior data mirroring and analysis. All patients participating in the GPeR gave their informed written consent for the scientific evaluation of their anonymized data prior use/entry of the GPeR and the study concept has been prospectively registered in the European Medicines Agency (EMA) registry for non-interventional/epidemiological studies (EUPAS identifier: 49718) to make this evaluation public.
Patients And Methods
Study design
PROVIDENCE is an exploratory, non-interventional, retrospective, comparative two-cohort-study using depersonalized data of the GPeR until May 31st, 2022, to assess the 4-week responder rate in comparable patient populations with a(L)BP and insufficient pain relief in response to self-medication who either received a first-line prescription with NSAIDs or alternatively with PRI.
Drugs under evaluation
NSAIDs are recommended by the German and various international guidelines for the first-line treatment of a(L)BP [14] and with annual prescription rates of 8.46 billion daily defined doses (DD) per year they represent the most frequently used and prescribed group of drugs for pain relief in the European Union [15]. NSAIDs exert their anti-inflammatory (and possibly also analgesic) effect by inhibiting so-called cyclooxygenases, which are responsible for the conversion of arachidonic acid into the prostaglandin PGe2 as well as a whole series of other/various subsequently formed prostaglandins. From a pathophysiological point of view, these prostaglandins are involved in the formation of the typical cardinal phenomena of inflammation, but also play a major role in the regulation of numerous and sometimes vital physiological processes, which is why the inhibition of their synthesis is often accompanied by unintended side effects [15,16].
Pridinol (PRI) a nonbenzodiazepine antispasmodic acting via cholinergic antagonism of muscarinic acetylcholine (M1) receptors [18], that has been reauthorized in Germany for the treatment of central and peripheral muscle spasms, torticollis, lumbago, and general muscle pain in adult patients end of 2017 and first time approved in 2020 in further European countries such as the United Kingdom, Poland, and Spain-proves very popular in daily practice, with continuously increasing prescription numbers and is currently one of only two antispasmodics approved for the treatment of peripheral muscle spasms associated with (L)BP in Germany [19]. Results from a recent meta-analysis of randomized controlled trials confirmed a significantly better pain relief, and improvement of function, muscle stiffness and rigidity for PRI vs. placebo associated with a comparable safety profile [14], and an analysis of data from the German Pain e-Registry (real world database) confirmed these results for daily practice as well [15], however, comparative efficacy and tolerability data vs. NSAIDs are yet not available. Due to its anticholinergic effect, the use of PRI is associated with a certain anticholinergic burden and thus a certain risk of peripheral (e.g. dry mouth, blurred vision, constipation, tachycardia, and urinary retention) or central side effects (e.g. writing confusion, dizziness, and cognitive impairment) [20,21], although both the currently available data from the two clinical studies mentioned before and the non-interventional analysis of available everyday data on practical use, as well as a recently published meta-analysis (based on the results of which PRI was assessed as a so-called “low potency drug”) [22], do not provide any evidence that the risk of side effects associated with the use of PRI is increased to any significant extent if the dosage recommendations of the current product information are observed [19].
Data source
This study used anonymized data from the German Pain e-Registry (GPeR) a national web-based pain treatment registry developed by the Institute of Neurological Sciences (IFNAP; Nürnberg) on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin, DGS eV) and the German Pain League (Deutsche Schmerzliga, DSL eV), which collects and aggregates individualized patient information, within a daily practice setting via its electronic front-end application iDocLive® a standardized electronic documentation platform.
The GPeR has been developed to provide patients and physicians with a standardized electronic documentation program to gather and evaluate clinically relevant patient information during daily routine practice and has been made available to all members of the German Pain Association, and patients, free of charge since 2014. Data were prospectively self-documented by patients using electronic case report forms of validated instruments as recommended by the German Pain Association and the German Pain League. The selection of the instruments as well as the survey times were determined by the treating physicians, considering the individual characteristics of the respective treatment case. Patient-reported data were electronically checked, analyzed, and supplemented by related physician information where appropriate and needed. Core parameters of the GPeR are based on the German Pain Questionnaire, and the German Pain Diary [23] two complex instruments that were mutually agreed upon by the German pain specialist societies and pain patient organizations as a standard for both quality-assured pain therapy as well as corresponding service remuneration by statutory and private health insurance companies [24] which cover a broad spectrum of validated instruments for the assessment of patient demography, history, pain characteristics (including pain type and phenomenology, pain intensity, severity, and stage of chronification, pain-related functional restrictions of daily life activities, quality of life, overall well-being, depression, anxiety, and stress, etc.), previous and current treatments, treatment response, and drug-related adverse events (DRAEs). For the purpose of this evaluation a depersonalized subset of the complete database of the GPeR has been used that consisted only of those parameters and patient data necessary for cohort matching as well as the evaluation of efficacy, safety and tolerability as described below.
Determination of sample size
There was no formal sample size calculation for this analysis. All patient data sets for whom the diagnosis and the treatment with the index medication have been newly initiated between January 1st, 2018, and May 31st, 2022, were generally available for this analysis and data sets were selected for both study cohorts according to the defined in- and exclusion criteria and a specific biometric matching process to ensure comparable baseline situations (see below). Treatment initiation was defined as no index medication use in the prior 12 weeks, and the date of first dose of treatment was set as the starting date for the definition of the 4-week evaluation periods. Analgesic treatments followed medical requirements according to the mutual/shared decisions of the participating physicians and patients and based exclusively on individual patient needs without any external specifications or influence.
In- and exclusion criteria
Inclusion criteria for patient data selection were: (a) patients with a diagnosis of a(L)BP, in whom (b) a treatment with either PRI or NSAIDs has been initiated for the first time (index date), by the current treating physicians based on individual patient needs (including but not restricted to decrease in functional status/activity or increase in pain intensity or intolerance to or ineffectiveness of prior self- treatments, etc.), and who (c) had a complete documentation with respect to all parameters necessary for evaluation at baseline and for the full follow up period of at least 4 weeks after the first treatment index medication. Exclusion criteria were: (a) a current diagnosis of cancer; (b) an already established diagnosis of c(L)BP; (c) previous spine surgery; (d) concomitant use of other prescription analgesics during the 4- week evaluation period.
Cohort pair matching
Data sets identified for this analysis were stratified according to the drug-treatments under evaluation: cohort A: PRI, cohort B: NSAIDs. A propensity score (PS) model was developed by which treatment status (PRI vs. NSAID) was regressed on observed baseline characteristics. The estimated propensity score for a patient was the predicted probability of treatment with either PRI or NSAID from the fitted regression model. Baseline characteristics included age, gender, average 24-hr pain- intensity (VAS) and pain-related disabilities (VAS) at baseline, duration of current a(L)BP symptoms, pain severity grading (due to von Korff), stage of pain chronification (due to the Mainz Pain Staging System, MPSS) comedications (ATC, first 3 digits), indications/diagnosis for treatment, previous and current self-medication (ATC, first 3 digits and/or medication group). Populations were matched via propensity score matching (PSM) procedures (nearest neighbor technique without replacement, caliper 0.15). Data sets of patients that were not able to be matched were excluded from further analysis. A comparison of the distribution of the baseline characteristics was performed to confirm the comparability of the selected patient cohorts before and after PSM prior biometric data evaluation [25].
General considerations
Data evaluations were performed for the subset of anonymized data provide by the GPeR according to the given in-/exclusion criteria and the criteria for the responder analysis and followed a modified intent-to-treat (ITT) approach as any data of patients who (a) took/recorded at least one dose of the index medications under evaluation and (b) recorded at least one post-baseline/post-dose measure were evaluated. As changes from baseline to endpoint were assessed, patient datasets were included in the analysis only if there was a baseline and a corresponding postbaseline measure within the 4-week evaluation period. All outcomes were summarized descriptively for baseline as well as end of week 4, and absolute and relative change from baseline using appropriate summary statistics and/or frequency distributions. Safety analyses were conducted on the safety analysis set, which included data of all patients who recorded to use at least one dose of the drugs under evaluation.
Descriptive and inferential statistical analyses were performed as reported. For continuous variables, descriptive statistics were summarized by the number of patients (n), the mean, standard deviation (SD), 95% confidence intervals (95%-CI) of the mean, median, and range (minimum-maximum) values. For categorical and ordinal variables data were summarized by frequency number (n), percentage (%) and (where appropriate) adjusted percentage (a%) of participants in each category, incl. 95% confidence intervals. For between groups comparisons of 2x2 contingency tables with a dichotomous/binomial trait McNemar´s tests (with the Edwards corrections) were applied, and Pearson’s chi-squared tests were used for categorial variables with multinomial expressions. Between groups comparisons of continuous variables were applied dependent on the data distribution: for normally distributed data paired samples t-tests and for non-normal distributions Wilcoxon´s signed rank test was performed. Where appropriate odds ratio (OR) as well as relative risks (RR; both ±95% confidence intervals), the numbers needed to treat/harm (NNT/H), and appropriate effect size (ES) measures (Cohen´s d, phi coefficient) were calculated [26]. All statistical tests were carried out using a 2-sided significance level of 0.05. Test results were presented as concrete p scores down to a level of 0.001, lower p scores were expressed as “≤0.001”. Since all comparisons were classified as exploratory, significance levels were not adjusted for multiplicity. Analyses were conducted using PASW Statistics (Version 18.0); tables and graphs (if appropriate) were built/rendered with Microsoft Excel (MS Office 365, Version 1911).
Handling of missing data
In the case of missing data, appropriate procedures were applied depending on the respective cause of missing to complete data sets. A baseline observation carried forward (BOCF) imputation was performed in case of missing data sets of patients who discontinued treatment due to a DRAE, death, or lack of efficacy, and a LOCF imputation (i.e. the last non-missing post baseline observation was carried forward to the corresponding endpoint for evaluation) has been performed for all other cases of missing data.
Primary outcomes
The proportion of patients classified as responder was the
primary outcome variable and was compared between both
evaluation cohorts (PRI and NSAIDs) on the basis of a composite
of five distinct response dimension (pain intensity, pain-related
disability, physical and mental quality-of-life, drug safety), known
to be relevant for a(L)BP patients and daily practice decisions.
Acute (L)BP intensity was rated on a 100-mm visual analogue scale
(VAS) with 0 = no pain to 100 = worst imaginable pain. The pain
intensity index (PIX) was calculated as the arithmetic mean of the
lowest, medium, and highest 24-hr. pain intensities documented by
patients [27]. Pain related impairments in daily activities related
to ‘home and family,’ ‘recreation,’ ‘social activities,’ ‘occupation,’
‘self-care/personal maintenance,’ ‘sleep’ and ‘overall quality of
life’ were reported using a modified version of the original pain
disability index (mPDI) [28] on a 100-mm VAS (0 = none to 100
= worst imaginable) to harmonize the different instrument scales.
Health-related quality of life was assessed with the eight physical
and mental domains of the Veterans Rand Short Form 12 (VR-
12) questionnaire [29, 30] and summarized in a physical and a
mental component score (PCS/MCS). Dimension specific response
definitions based on clinically relevant/meaningful improvements
at end of week 4 vs. baseline and were defined as follows:
• an improvement of the average 24-hr. pain intensity index
≥20mm VAS (the minimum clinical important difference, MCID)
or ≥50 percent [31]
• an improvement of the pain-related disability (as assessed
with the modified pain disability index, mPDI) ≥20mm VAS
(MCID) or ≥50 percent [23]
• an improvement of the physical quality-of-life (as assessed
with the VR-12 physical component subset, PCS) >3.77 points
(MCID) [32]
• an improvement of the mental quality-of-life (as assessed
with the VR-12 mental component subset, MCS) >3.29 points
(MCID) [33]
• no premature DRAE-related treatment discontinuation.
Primary endpoint analysis
For the purpose of this study, a responder has been defined as a patient who met all of the response criteria mentioned above at the end of the 4-week evaluation period after start of the index medication. For the calculation of the primary endpoint a sequential non-inferiority - superiority analysis was performed. For this purpose, non-inferiority of cohort A (PRI) vs. cohort B (NSAIDs) was tested in a first step and confirmed if the lower bound of the 95% CI of the primary response rate for cohort A was above the lower bound of the corresponding 95%-CI for cohort B. In case noninferiority has been confirmed and if statistical analyses indicate either a significant (p score < 0.05) as well as clinically relevant (Cohen´s d/Phi > 0.2) between cohort difference in favor of cohort A (PRI) vs. cohort B (NSAIDs), a superiority analysis followed in a second step and superiority was confirmed if a) the 95% CI of the primary outcome response rate of both treatment cohorts did not overlap, b) none of the individual response criteria contradicts this difference, and c) the number of patients with DRAEs and those with DRAE-related treatment discontinuations in cohort A (PRI) was significantly lower than those documented for cohort B (NSAIDs).
Secondary efficacy outcomes
Secondary efficacy analyses were done with respect to the absolute/relative change of the four efficacy response dimensions at end of week 4 vs. baseline.
Safety and tolerability analyses
Safety was assessed by summarizing and analyzing the frequency and spectrum of DRAEs, the number of patients with DRAEs, as well as DRAE-related treatment discontinuations by treatment group and for the 4-week evaluation period of this study. For the purpose of this study, DRAEs have been defined as injuries/ events that were (a) newly reported or reported to worsen in severity after the initiation of index medications under evaluation, and (b) for which a causal relationship with one of the two index therapies was assumed.
Extent of exposure
Treatment exposure was defined as the time from the date when the index medication has been taken for the first time until the last recorded treatment dose within the 4-week evaluation period. Average daily dosages were analyzed for each index medication in mg as well as referenced to the daily defined dosages (DDD) reported in the current Anatomical Therapeutic Chemical (ATC) classification [34].
Data minimization principle
As requested by the General Data Protection Regulation (GDPR) of the European Union (EU) [35], the use of depersonalized data for the purpose of this study was adequate and followed the data minimization principle, i.e. data selected and processed were not held or further used unless it was essential for reasons that were clearly stated in advance to support data privacy. Therefore, only those parameters necessary for the analyses and procedures described in this document (especially those necessary to answer the primary and secondary outcomes as well as supplemental topics) were extracted for this study and the evaluation described.
Maintenance / provisioning of raw/original data
According to the current standard operating procedures of the GPeR and legal specifications (EU-GDPR), the results of any analyses that based on depersonalized data extracted from the GPeR were provided only in aggregated tables/figures without any information on individual patient scores or participating pain centers. All data extracted from the GPeR for the purpose of this study will be kept until three months after the complete completion of all study-specific services and reporting procedures specified in the respective statistical analysis plan for this study and will then be completely deleted. Due to specific SOPs of the GPeR, there is currently no possibility of sharing raw data for secondary analyses that have not been described in advance in the corresponding study plan.
Results
Study cohorts
As a result of the propensity score-based matching process, the data sets of 467 patients per cohort were selected for this analysis (Figure 1). Patients in cohort A received a treatment with PRI, while those in cohort B received a treatment with different NSAIDs such as ibuprofen (n=241), diclofenac (n=187), naproxen (n=24), meloxicam (n=9), or others (n=6)-each after documented failure of self-medication and/or non-pharmacological self-management strategies.
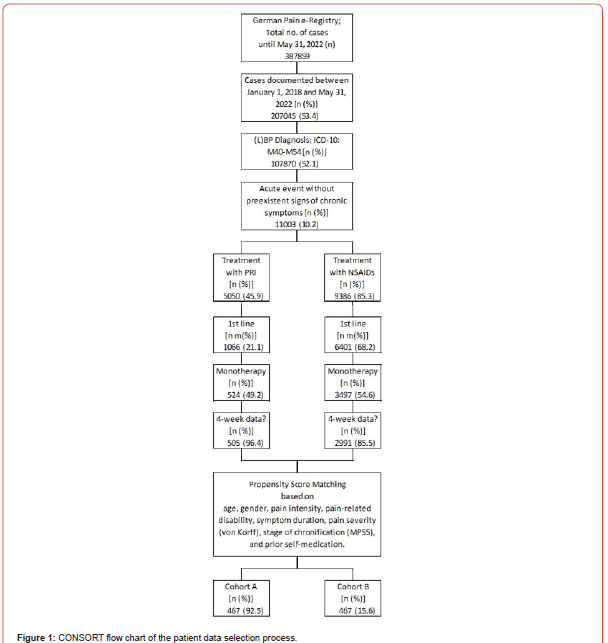
Patient demographics and non-pain baseline characteristics
Details of demographics and baseline characteristics are summarized in (Table 1). Average age at onset of treatment was 56.4±10.8 (median 57, range 28-82) years, and 55.5% (n=259) of patients were female. For 371 patients per cohort (79.4%), a(L)BP diagnosis was categorized as ICD-10 M50-M54 (“other diseases of the spine and back”), for 58 patients (12.4%) as M45-M49 (spondylopathy”), and for 38 as M40-M43 (“deformities of the spine and back”). With 90.4%, nine of ten patients suffered from acute pain according to stage I of the Mainz Pain staging System (MPSS). Disease intensity was high with 68.3% of patients belonging to grade III or IV of the von-Korff pain intensity grading score. Average duration of the a(L)BP episode was 13.7±9.6 vs. 13.8±9.8 days for patients of cohort A (PRI) vs. B (NSAIDs) with a median of 11 and a range of 1-42 days in both cohorts (p=0.901, ES: 0.003). Comorbid conditions were frequent and reported by the majority of patients, only 54 patients in each cohort (11.6%) reported none. Average number of comorbidities was 1.9±1.2 (median 2, range 0-6 vs. 0-7) in study cohorts A vs. B (p=0.979, ES: 0.000). Clinical risk factors for the use of NSAIDs (e.g. gastrointestinal-problems, cardiovascular and renal comorbidities, diabetes, etc.) were highly prevalent and documented by 315 vs. 322 patients in cohorts A vs. B (p=0.972, ES: 0.000). On average data sets selected for this analysis contained information on the average use of 1.4±1.0 (median 1, range 0-4) pharmacological comedications. Pharmacological risk factors for the use of NSAIDs (e.g. use of antihypertensive drugs, antithrombotic agents, corticosteroids, etc.) were prevalent as well and reported by 280 vs. 273 patients in cohorts A vs. B (p=1.0; ES: 0.000). In combination with an age of 65 years (or higher), 78.4 vs. 79.4 percent of patients (n= 366 vs. 371) in cohorts A vs. B documented at least one clinical and/or pharmacological risk factor for the use of NSAIDs (p=0.883; ES: 0.013). As requested by the inclusion criteria, all patients in both cohorts documented the use of OTC-NSAIDs (on average 1.4±0.5), and 146 vs. 148 in cohorts A vs. B (31.3 vs. 31.7%) also reported the use of different other systemic OTC-analgesics (on average 0.3±0.5 for both cohorts) prior use of the index medications (Table 2). Topical application of NSAIDs was reported by 162 vs. 163 patients in cohort A vs. B (34.7 vs. 34.9%; p=ns) and the execution of non-pharmacological treatment strategies was reported by 178 patients in both cohorts (38.1%; p=ns).
Table 1:Demographic and non-pain baseline characteristics.
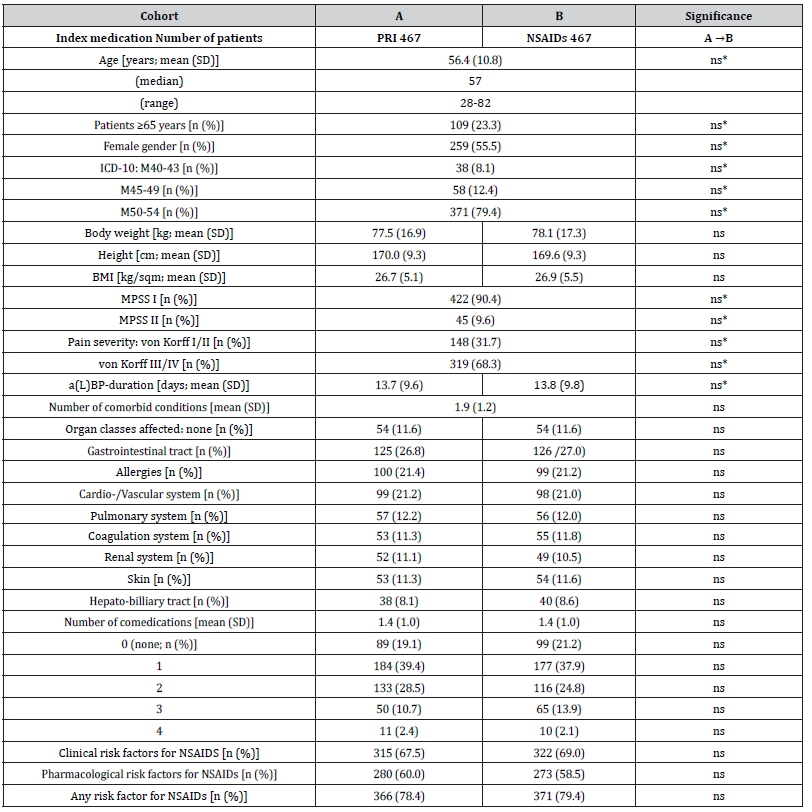
Notes: SD: standard deviation; BMI: body mass index; MPSS: Mainz pain staging system; a(L)BP: acute (low) back pain; NSAIDs: nonsteroidal anti-inflammatory drugs. Parameters marked with an asterisk (*) are those chosen for the propensity score matching process.
Table 2:Overview over OTC self-medication prior prescription of index treatments.
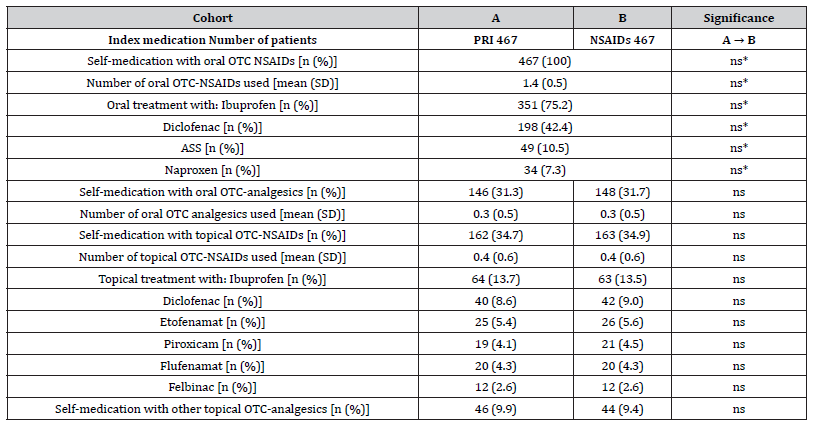
Notes: OTC: over the counter; NSAIDs: nonsteroidal anti-inflammatory drugs. Parameters marked with an asterisk (*) are those chosen for the propensity score matching process.
Study medication
The average daily dose of PRI was 8.25±1.6 (median 9, range 4.5-13.5) mg. 91.9% of patients (n=429) reported a daily dose of 9 mg (the recommended daily dose) or less, only 8.1% (n=38) of dosages above the recommended daily upper limit. The most common NSAID used for treatment was ibuprofen (n=241), followed by diclofenac (n=187), naproxen (n=24) and meloxicam (n=9). Average dosages varied dependent on the distinct NSAID and were 1736.1±291.1 (median 1800, range 1200-2400) mg for ibuprofen, 111.2±21.0 (median 100, range 50-150) mg for diclofenac, 541.7±256.9 (median 500, range 250-1000) mg for naproxen, and 12.9±2.6 (median 15, range 7.5-15) mg for meloxicam. A harmonized-DDD-referenced – dose analysis for each drug under evaluation-revealed a significantly lower percentage of patients in whom the dosages given were above the DDD (8.1%) in cohort A (e.g. those treated with PRI) in comparison to the comparative treatments of patients in cohort B (NSAIDs), where 33.3% of patients documented daily dosages above the respective DDD. DDD-referenced relative daily dosages were 91.7±18.2% (median 100) for PRI vs. 144.7±24.3% (median 150) for ibuprofen, 111.2±21.0% (median 100) for diclofenac, 108.3±51.4% (median 100) for naproxen, and 88.9±12.4% (median 100) for meloxicam.
Treatment response
Average 24-hr. pain intensity index (PIX). Relevant information on the PIX is aggregated in (Table 3). Based on the patients’ information on their lowest, medium and maximum 24-hr. pain intensities, the average 24-hr. pain intensity index (PIX) was calculated to be at baseline 58.8±7.4 mm VAS for patients in both cohorts (p=1.0) and dropped down to 15.9±5.6 mm VAS in response to treatment with PRI (cohort A; p<0.001, ES: 1.650) vs. 21.9±6.3 mm VAS with NSAIDs (p<0.001, ES: 1.347). Absolute improvement was 42.9±7.4 vs. 36.9±8.5 mm VAS in cohort A vs. B (p<0.001; ES: 0.189), corresponding to a relative improvement of 73.0±16.4 vs. 62.8±22.8 percent vs. baseline (p<0.001; ES: 0.130). An absolute improvement equal to or even greater than 20 mm VAS at end of week 4 vs. baseline was documented by 88.4 vs. 77.9% of patients in cohort A vs. B (n=413 vs. 364; p=0.085, ES: 0.140), and a relative improvement equal to or even greater than 50% vs. baseline at end of week 4 was reported by 83.5 vs. 72.8% (n=390 vs. 340; p=0.070, ES: 0.130). The combined response (i.e. either an absolute PIX improvement ≥MCID and/or a relative PIX improvement ≥50% at end of week 4 vs. baseline, was found for 88.7 vs. 78.2% of patients in cohort A vs. B (n=414 vs. 365; p=0.085, ES: 0.141).
Table 3:Baseline and end of week 4 scores (incl. absolute and relative changes vs. baseline) for the average 24-hr. a(L)BP intensity index (PIX)
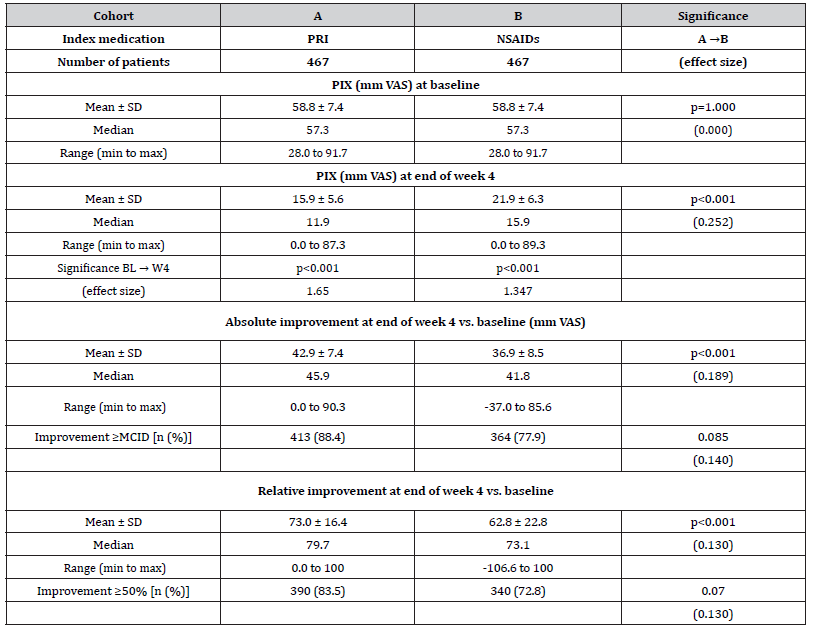
Notes:PIX: average 24-hour pain intensity index; VAS: visual analogue scale; SD: standard deviation; min: minimum; max: maximum; MCID: minimal clinical important difference.
Pain-related disabilities in daily life (mPDI)
Details on the mPDI score at baseline as well as end of week 4 with index medication can be found in (Table 4). The a(L)BPrelated disability with respect to daily life activities at baseline was identical in both cohorts (63.6±8.1 mm VAS; median 64.1, range 29.1-97.0; p=1.0) and improved to 19.7±8.0 (median 16.8, range 0.0-85.9) mm VAS in patients of cohort A vs. 36.9±9.9 (median 31.1, range 1- 99.6) mm VAS in patients of cohort B (p<0.001; ES: 0.480). Absolute improvement was 44.0±9.1 (median 45.2, range 0.0-85.6) mm VAS vs. 26.8±13.1 (median 28.8, range -67.6 to 85.9) mm VAS in cohort A vs. B (p<0.001; ES: 0.387), corresponding to a relative improvement of 69.0±16.6 (median 73.2, range 0-100) vs. 42.0±30.2 (median 47.6, range -221.8 to 98.7) percent vs. baseline (p<0.001; ES: 0.289). An absolute improvement ≥20 mm VAS at end of week 4 vs. baseline (the MCID for the mPDI) was documented by 90.4 vs. 61.0% of patients in cohort A vs. B (n=422 vs. 285; p<0.001, ES: 0.342) and a relative improvement ≥50% vs. baseline at end of week 4 was reported by 91.0 vs. 48.0% (n=425 vs. 224; p<0.001). Overall, 91.0 vs. 61.2 percent of patients in cohort A vs. B (n=425 vs. 224) recorded a combined response [i.e. either an absolute mPDI improvement ≥MCID and/or a relative mPDI improvement ≥50% vs. baseline; p<0.001, ES: 0.349, OR: 6.4 (95%-CI: 4.4-9.3), RR: 3.2 (95%-CI: 3.0-3.4), NNT 3.4].
Table 4:Baseline and end of week 4 scores (incl. absolute and relative changes vs. baseline) for a(L)BP-related disabilities (mPDI).
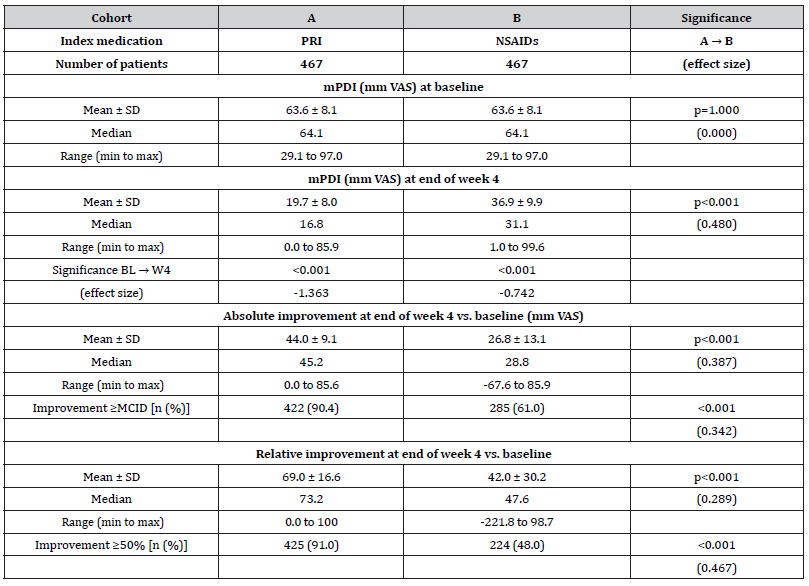
Notes:mPDI: modified pain disability index; VAS: visual analogue scale; SD: standard deviation; min: minimum; max: maximum; MCID: minimal clinical important difference.
Physical quality of life (VR12-PCS)
The VR12-PCS scores at baseline were reported to be 35.3±7.1 (median 35.0, range 19.1-59.7) for patients in cohort A vs. 35.2±6.7 (median 34.6, range 19.8-59.3) for those in cohort B (p=0.902; see overview in (Table 5) and increased to 45.8±6.5 (median 46.0, range 20.1-72.7) in response to treatment with PRI (cohort A) vs. 41.6±6.1 (median 42.0, range 20.5-71.3) with NSAIDs (p<0.001; ES: 0.167). Absolute improvement was 10.5±3.2 (median 12.0, range -1.0 to 16.0) vs. 6.4±2.7 (median 6.0, range -1.0 to 14.0) in cohort A vs. B (p<0.001; ES: 0.347). An absolute improvement equal to or even greater than 3.77 points at end of week 4 vs. baseline (the MCID for the VR12-PCS) was documented by 83.1 vs. 70.0% of patients in cohort A vs. B [n=388 vs. 327; p=0.025, ES: 0.154, OR: 2.1 (95%-CI: 1.5-2.9), RR: 1.5 (95%-CI: 1.4-1.6), NNT 7.7].
Table 5:Baseline and end of week 4 scores (incl. absolute and relative changes vs. baseline) for the physical quality of life (VR12-PCS).
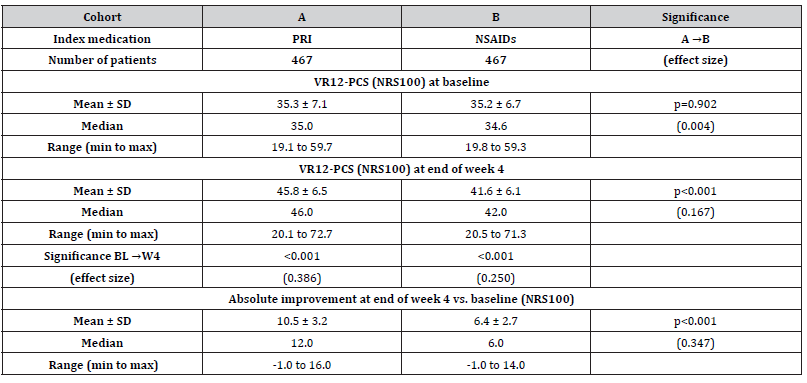
Notes:VR12-PCS: physical component scale of the 12 questions short form of the Veterans Rand quality-of-life questionnaire; NRS: numerical rating scale; SD: standard deviation; min: minimum; max: maximum.
Mental quality of life (VR12-MCS)
Baseline scores of the VR12-MCS score were 41.6±7.1 (median 41.7, range 16.1-65.6) vs. 41.6±7.3 (median 42.2, range 15.3-64.9) for patients in cohort A vs. those in cohort B (p=0.986), (Table 6) and improved to 49.3±8.0 (median 48.8, range 20.0-75.6) vs. 47.2±8.2 (median 48.0, range 20.0-74.4; p=0.004; ES: 0.065). Absolute improvement was 7.7±4.4 (median 9.0, range -2.0 to 13.0) in cohort A vs. 5.6±4.1 (median 6.5, range -3.0 to 13.5) in cohort B (p<0.001; ES: 0.124). An absolute improvement equal to or even greater than 3.29 points at end of week 4 vs. baseline (the MCID for the VR12-MCS) was documented by 77.7 vs. 63.0% of patients in cohort A vs. B [n=363 vs. 294; p=0.008, ES: 0.162, OR: 2.1 (95%-CI: 1.5-2.7), RR: 1.5 (95%-CI: 1.4-1.5); NNT 6.8].
Table 6:Baseline and end of week 4 scores (incl. absolute and relative changes vs. baseline) for the mental quality of life (VR12-MCS)..
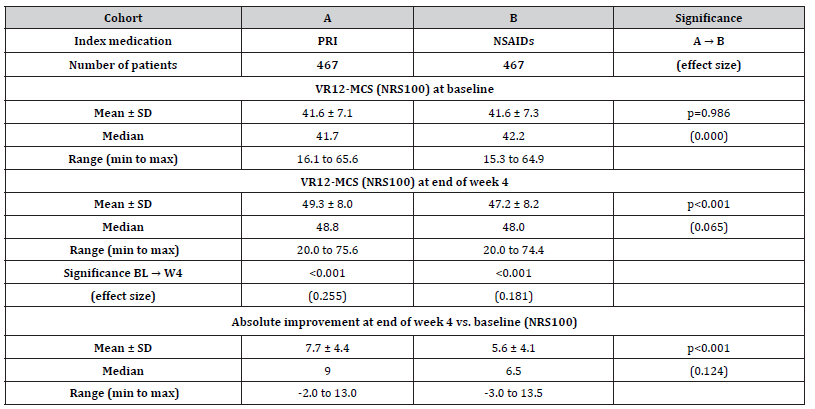
Notes:VR12-MCS: mental component scale of the 12 questions short form of the Veterans Rand quality-of-life questionnaire; NRS: numerical rating scale; SD: standard deviation; min: minimum; max: maximum.
Premature treatment discontinuations
As a direct reaction to DRAEs, 3.4 vs. 9.4% of patients (n=16 vs. 44) discontinued treatment with PRI vs. NSAIDs [p<0.001; ES 0.122, OR: 0.3 (95%-CI: 0.2-0.6), RR: 0.5 (95%-CI: 0.3-0.9); NNH 16.7] and 8.6 vs. 27.6% (n=40 vs. 129) discontinued treatment due to inadequate effectiveness [p<0.001; ES: 0.248, OR: 0.3 (95%-CI: 0.2- 0.4), RR: 0.4 (95%-CI: 0.3-0.6); NNH 5.3], resulting in a combined premature discontinuation rate of 12.0 vs. 37.0% of patients in cohort A vs. B [n=56 vs. 173; p<0.001; ES: 0.291, OR: 0.2 (95%-CI: 0.2-0.3), RR: 0.4 (95%-CI: 0.3-0.6); NNH 4.0]. In addition, 13.7 vs. 4.7% of patients in cohort A vs. B. (n=64 vs. 22) stopped treatment prior end of week 4 as a consequence of a significant relief or even complete vanishing of a(L)BP symptoms [p<0.001, ES: 0.156, OR: 3.2 (95%-CI: 1.9-5.3), RR: 1.6 (95%-CI: 1.0-2.5); NNT 11.1].
Safety and tolerability analysis
All in all, 42 vs. 97 patients within cohort A vs. B (9.0 vs. 20.8%) reported at least one DRAE (p<0.001, ES: 0.165, OR: 0.4 (95%-CI: 0.3-0.6), RR: 0.6 (95%-CI: 0.4-0.8); NNH 8.5). Average timepoint of DRAE experience was 14.4±7.0 (median 14, range 1-28) vs. 15.5±7.7 (median 16, range 2-28) days after treatment initiation in cohort A vs. B (p=0.323, ES: 0.045). Timepoint of DRAE experience and those of DRAE-related premature treatment discontinuation for patients in both cohorts are shown in (Figures 2 & 3). Spectrum of DRAEs reported is shown in (Table 7). Overall, 51 vs. 136 DRAEs were documented by patients who received PRI vs. NSAIDs. Significant between cohort differences – all in favor of PRI vs. NSAIDs – were found for gastrointestinal DRAEs (17 vs. 82, p<0.001) such as abdominal pain (5 vs. 29, p<0.001), dyspepsia (0 vs. 18, p<0.001), diarrhea (0 vs. 15, p<0.001), and vomiting (0 vs. 10, p<0.001), decreased appetite (n=0 vs. 8, p=0.013), and peripheral oedema (0 vs. 6, p=0.041). Insignificantly higher DRAE rates with PRI vs. NSAIDs were seen for dry mouth (6 vs. 1), headaches (17 vs. 12), somnolence (4 vs 1), and muscular weakness (3 vs. 0), circulatory instability (3 vs. 1), and diastolic hypotension (2 vs. 0), whereas nausea (6 vs. 9), dizziness (4 vs. 7), tachycardia (1 vs. 4), and rash (0 vs. 5) were numerically more prevalent with NSAIDs.
Table 7:Summary of drug-related adverse events (DRAEs) reported during the 4-week treatment evaluation for a(L)BP patients treated either with PRI or NSAID.
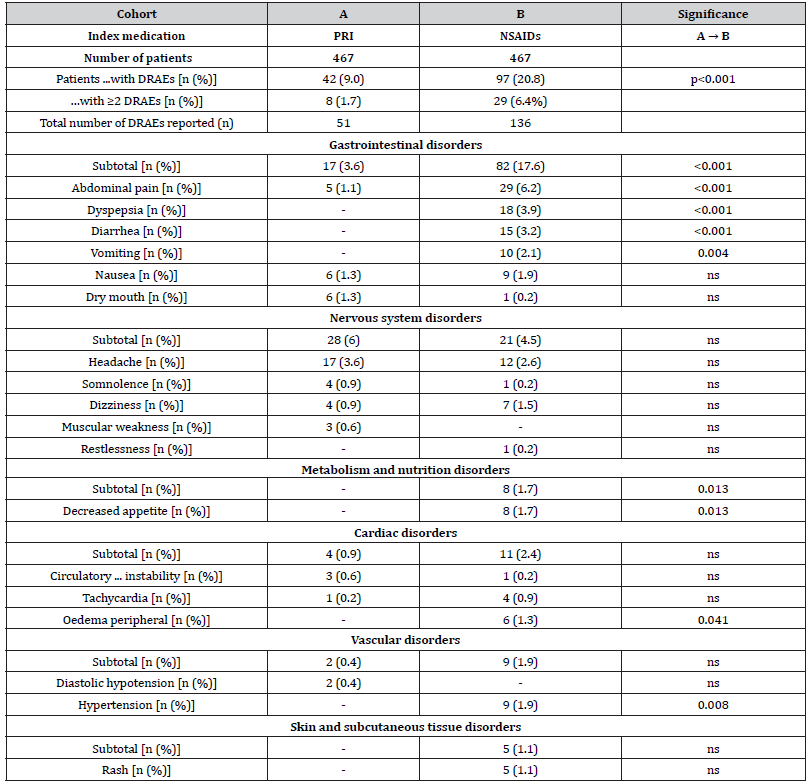
Notes:DRAEs: drug-related adverse events.
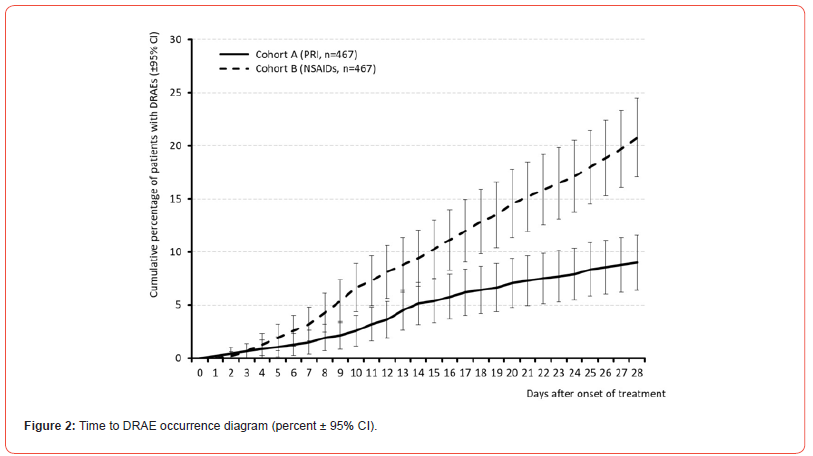
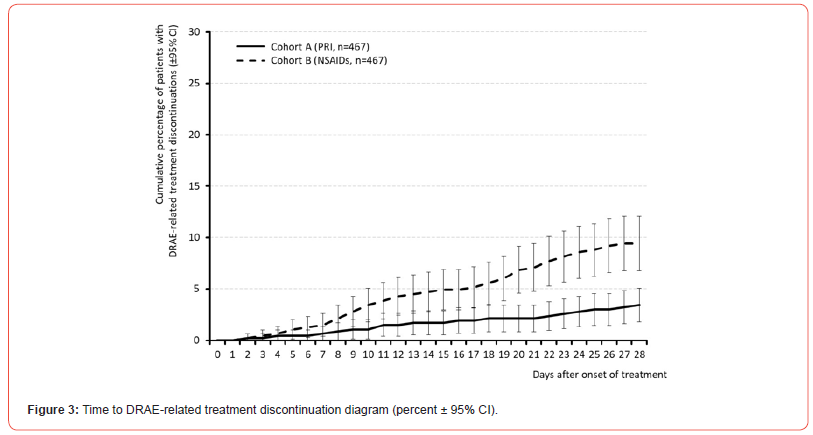
Primary endpoint analysis
All in all, 322 vs. 159 patients in cohort A vs. B [69.0 (95%- CI: 66.0-71.9) vs. 34.0 (95%-CI: 31.0-37.1) percent] reached the primary endpoint of this analysis [p<0.001, ES: 0.349, OR: 4.3 (95%-CI: 3.3-5.7), RR: 2.1 (95%-CI: 1.8-2.4); NNT 2.9; see (Table 8). Out of the six individual efficacy criteria necessary to qualify as a responder, patients in cohort A vs. B achieved on average 5.1±1.7 (median 6) vs. 3.9±2.0 [median 4; p<0.001; ES: 0.162, OR: 7.4 (95%-CI: 5.4-10.2), RR: 3.2 (95%-CI: 3.0-3.5): NNT: 2.5]. (Figure 4) shows the risk ratios of response for patients in cohort A (PRI) vs. B (NSAIDs) for all components of the primary endpoint as well as the combined endpoint aggregate. For all between cohort comparisons the lower boundaries of the 95%-CIs were clear above 1 with highest between cohort response differences found for mPDI, the parameter evaluating functionality and pain-related disabilities in daily life activities.
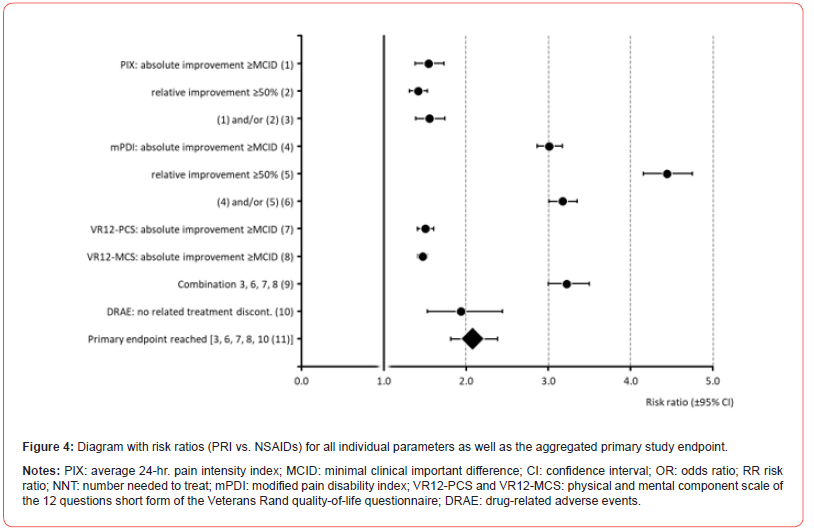
Table 8:

According to the sequential non-inferiority/superiority evaluation concept, treatment of a(L)BP with PRI proved not only non-inferior [lower bound of the 95% CI of the primary outcome response rate for cohort A (66.0%) was above the lower bound of the corresponding 95%-CI for cohort B (31.0%)] but even superior to the treatment with NSAIDs, because not only was the aggregate primary endpoint significantly (p<0.001) as well as clinically relevant (ES: 0.349) different between the two treatments groups (and in favor of PRI), but also a) the 95% CIs of the primary endpoint did not overlap, b) all individual components showed better results for PRI vs. NSAIDs, and c) both the number of patients with DRAE and those with DRAE-related study discontinuations were significantly lower with PRI than with NSAIDs.
Discussion
a(L)BP are widespread, frequent, and associated with a high risk of recurrence or chronicity. Current guideline recommendations regarding the preferred use of NSAIDs do not adequately address the muscular causes underlying the majority of cases, as these drugs act purely symptomatic and lack any direct effect on the increased muscle tension frequently seen in patients with a(L)BP. Due to their non-specific mechanism of action and its fundamental importance for numerous physiological body functions, NSAIDs are rightly considered problematic, not only for elderly patients and those with clinical and/or pharmacological risk factors, but also for long-term use in doses that are therapeutically effective.
The justification for the preferred positioning of NSAIDs in the treatment of a(L)BP is a more favorable data situation - compared to other forms of therapy (e.g. antispasmodics), whereby the extent of the significant efficacy differences demonstrated in the clinical studies compared to placebo is not only frequently only of subclinical relevance for the patients, but the underlying efficacy data were regularly evaluated separately from the safety and tolerability data, which is why ultimately a discordant picture results for the practical routine of care. This study is the first to evaluate and demonstrate the efficacy of the antispasmodic PRI in comparison to NSAIDs as first line prescription for patients with a(L)BP under conditions of daily life. Over the evaluation period of 4 weeks, both treatment alternatives proved to be effective in that we observed a significant decrease in pain intensity and painrelated functional restrictions vs. baseline for both study cohorts. However, with the antispasmodic the proportion of patients who finally met the primary study endpoint was twice as high as with NSAIDs, which led to the result that PRI scored not only noninferior compared to those NSAIDs that have been evaluated in our study, but even superior.
Main driver of these between treatment differences was the significantly stronger decline of a(L)BP-related functional restrictions in daily life activities with PRI in conjunction with the significantly lower discontinuation rate in response to the dissatisfaction of patients with the analgesic effect and its better safety and tolerability profile as demonstrated by the significantly lower rate of DRAEs and DRAE-related treatment discontinuations. The significantly greater relief of muscle-related functional limitations also influenced the between-group differences in the other three efficacy parameters that we´ve evaluated, where patients who received PRI showed also stronger improvements vs. those with NSAIDs, but slightly less marked in comparison to the response seen for the physical function dimension. Despite the short evaluation period of only four weeks, 37.0% of patients on NSAIDs discontinued treatment prematurely-either because of DRAEs (9.4%) or because they were dissatisfied with the analgesic effect (27.6%), but only 12.0% with PRI (3.4% and 8.6%). This can be taken as an indication that a mechanism-based (in any case muscle-related) approach of PRI makes sense not only in theory, but especially in daily practice – at least when OTC-NSAIDs haven´t previously shown sufficient effect.
Treatment of a(L)BP with a non-benzodiazepine antispasmodic like PRI is also a rational alternative to NSAIDs when individual patient profiles show significant risk factors that are considered either a warning signal or even a concrete contraindication for the application of NSAIDs-which surprisingly was the case in eight out of ten patients in both cohorts in our study. Nevertheless, we found in our study that patients with a(L)BP were prescribed NSAIDs despite such pre-existing risk factors, which in our view raises some questions regarding the practical implementation of the current guideline recommendations regarding the prescription of NSAIDs, in which the small print is obviously often overlooked or read over. The effects reported do not only confirm the results of previous placebo-controlled studies on the efficacy and tolerability of PRI in various acute muscle pain disorders-in which PRI showed a significantly stronger effect vs. placebo at comparable tolerability [14] but also those of a recent Cochrane review which shows moderate evidence that NSAIDs are at best slightly more effective than placebo for short-term pain reduction in patients with a(L)BP, with effect sizes below clinical relevance [36-38].
These differences shown here are even more meaningful for daily practice simply due to the fact that in contrast to the segregated efficacy and safety/tolerability analyses performed in clinical trials, we aggregated these dimensions and evaluated them simultaneously in form of a composite endpoint, chosen to mirror common daily-practice approaches (where it ultimately does not matter whether a therapy fails because it does not work sufficiently strong or because it is not tolerated sufficiently well). Overall, the present results call not only physicians’ willingness/ability to adjust pharmacological options beyond NSAIDs depending on the specifics of the individual patient situation, but also the flexibility of guideline instructions to differentiate drug-specific variations between multiple muscle relaxants as required by the available pharmacological and biometric study data. For years, muscle relaxants and antispasmodics have been assessed as a uniform group in most guidelines and not only efficacy, but also safety and tolerability data of different components have been uncritically generalized.
Until 2022, no citable or assessable data existed for PRI, simply because the necessary information and study reports were neither systematically summarized nor published. This is ultimately the reason why this active ingredient is not even mentioned by name in any of the current (L)BP guidelines and this is mistakenly regarded as a negative recommendation for this nonbenzodiazepine antispasmodic with reference to the generally negative evaluation of muscle relaxants - especially benzodiazepines - in .. this indication. As a consequence, PRI prescriptions are criticized, and prescribers accused of unscientific/uneconomical behavior (frequently combined with the threat of regress claims), whereas the reflexive prescription of non-specific NSAIDs-despite evidently insufficient analgesic and function-improving effects, but numerous (and sometimes even deleterious) adverse effects-is tolerated uncritically.
The prevalence and spectrum of DRAEs we´ve evaluated in this study for PRI were consistent with those reported in the current prescribing information and in the aforementioned studies and did not indicate any new, serious, or persistent adverse health effects. With exception of the DRAE dry mouth, which was reported by 6 vs. 1 patient with PRI vs. NSAIDs, none of the known peripheral and central signs for anticholinergic effects was more prevalent with PRI compared to NSAIDs, which ultimately underlines previous observations that PRI is a safe and well-tolerated form of treatment when the recommended dosage is followed. As far as the evaluation of the treatment data available to us allowed, we were able to demonstrate that the documented DRAEs resolved completely, either spontaneously or after termination of treatment. This observation is also consistent with that of the clinical studies as well as the aggregated user experiences with PRI published by us, in which both the frequency and severity of DRAEs were low, did not necessarily lead to treatment discontinuation and which also regressed completely if the recommended daily dose (of 3 x 3 mg) was not exceeded for a longer period of time – which was the case in 91.9% of all patients in the present study.
Vice versa, the higher rate of DRAEs and DRAE-related treatment discontinuations seen with NSAIDs in the present study may also be a consequence of the prescription of higher daily dosages for these drugs, which were above the DDD of the various active agents in as many as one third of the patients in Cohort B. In this context, it is important to note that the non-specific (and thus for the majority of patients insufficient) effect of NSAIDs with regard to a(L)BP was probably largely responsible for the prescription of higher doses that we were able to show, which counteracted the current recommendations for the use of NSAIDs (“in the lowest possible dose for the shortest possible time”) and ultimately worsened the risk of patients. Ultimately, however, it must also be noted that NSAIDs - even in the form of short-term use evaluated by us in the present case - are not as harmless and well tolerated as usually assumed. Statistically, the between-group differences we found were primarily dominated by gastrointestinal DRAEs (which were documented almost 5 times more frequently under NSAIDs than under PRI). However, it is worth looking at the details of our DRAE analysis, which also confirmed striking differences for cardiovascular/renal DRAEs (such as peripheral oedema and hypertension) to the disadvantage of NSAIDs, which are known to (may) become increasingly important in the case of (not entirely uncommon) longer-term treatment.
Limitations
In view of the design of the present study, the retrospective analysis of depersonalized routine data from standard care, the lack of randomization and the lack of placebo comparison, the present analyses are of course accompanied by certain limitations compared to prospectively randomized placebo-controlled clinical trials and these weaknesses should be considered when interpreting our data and transferring them to the everyday treatment setting. On the other hand, however, there are also numerous advantages, such as the non-interventional character, the use of data from standard/ routine care, the lack of artificial/unrealistic framework conditions (as is common in clinical trials), the direct comparison with firstline pharmacotherapies applied in-line with inter-/national a(L)BP guidelines and the lack of external influence (e.g. through artificial inclusion/exclusion criteria and reimbursements of patients as well as physicians for participating in a controlled clinical trial, etc.).
From a scientific point of view, the most obvious weakness of our evaluation compared to clinical trials was the use of a so-called composite endpoint. However, this was at the same time also the main strength of our study approach, because while in randomized controlled trials the dimensions efficacy, safety and tolerability are usually considered as different endpoints and separate from each other, the true benefit of a drug in daily praxis is defined by the best possible combination of all these dimensions. This also applies to the guideline-compliant use of NSAIDs for the treatment of people with a(L)BP, which is ultimately not worthwhile if either (a) precautions and/or contraindications restrict their application in daily practice, (b) side effects occur and impair the patients’ well-being to such an extent that they are forced to discontinue treatment, or (c) those without DRAEs perceive neither clinically relevant pain relief nor a significant improvement in pain-related functional limitations in everyday life or in the physical and/or mental quality of life with the use of the drug. A further limitation results from the procedure we used to select the data sets suitable for this form of comparative retrospective analysis and the question of whether generalized statements can also be made with regard to the cases that were not part of the analysis (because they were excluded within the framework of the PSM procedure). While this question does not arise for the group of patients treated with PRI due to the high selection rate (92.5%), the almost 6-times larger base population of patients treated with NSAIDs and the subsequent lower selection rate (of only 15.6%) could well suggest that specific (and nonrepresentative) characteristics of the NSAID patients ultimately included in our analysis might be responsible for the described between-group differences (especially with regard to the poor tolerability data), which would then naturally not be generalizable in this way. However, the comparative analyses of demographic as well as baseline characteristics of selected vs. unselected NSAID patients that we conducted in this context confirmed the basic comparability of both collectives and did not reveal any indicators of specific selection phenomena. For this reason, we do not assume that procedure-related factors are responsible for the betweengroup differences described, but rather drug-specific differences.
The present study does not claim to replace a prospective double-blind randomized placebo-controlled trial. Rather, the aim of this study was (and is) to evaluate effectiveness and tolerability of two forms of therapy (the causally oriented use of an antispasmodic compared to the symptomatic use of NSAIDs) under the framework conditions of routine care in German practices/centers. Under this objective, the guideline-compliant use of NSAIDs in a(L)BP patients difficult to treat in other ways ultimately proved to be a reasonably effective but worse (than expected) tolerated treatment alternative, whereas the antispasmodic PRI proved to be not only superior effective vs. NSAIDs but also significantly better tolerated.
Summary
Based on the results of this propensity score matched retrospective analysis of the 4-week response and tolerability data of patients with a(L)BP refractory to self-medication, who received either the non-benzodiazepine antispasmodic PRI or guideline recommended NSAIDs, patients treated with PRI reported a significantly stronger improvement of pain-related disabilities, pain intensities, as well as physical and mental quality of life in combination with significantly less DRAEs and less DRAE-related treatment discontinuations, what results in a superior effectiveness of the antispasmodic PRI vs. NSAIDs.
Acknowledgment
None.
Conflict of Interest
No Conflict of Interest.
References
- Nasser MJ (2005) How to approach the problem of low back pain: an overview. J Family Community Med 12(1): 3-9.
- Borczuk P (2013) An evidence-based approach to the evaluation and treatment of low back pain in the emergency department. Emerg Med Pract 15(7): 1-23.
- Patel EA, Perloff MD (2018) Radicular pain syndromes: cervical, lumbar, and spinal stenosis. Semin Neurol 38(6): 634-639.
- Luciola da C Menezes Costa, Christopher G Maher, Mark J Hancock, James H McAuley, Robert D Herbert, et al. (2012) The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ 184(11): E613-624.
- C J Itz, J W Geurts, M van Kleef, P Nelemans (2013) Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain 17(1): 5-15.
- Amir Qaseem, Timothy J Wilt, Robert M McLean, Mary Ann Forciea, Thomas D Denberg, (2017) Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 166(7): 514-530.
- Tatiane da Silva, Kathryn Mills, Benjamin T Brown, Robert D Herbert, Christopher G Maher, et al. (2017) Risk of recurrence of low back pain: a systematic review. J Orthop Sports Phys Ther 47(5): 305-313.
- Jan Hartvigsen, Mark J Hancock, Alice Kongsted, Quinette Louw, Manuela L Ferreira, et al. (2018) What low back pain is and why we need to pay attention. Lancet 391(10137): 2356-2367.
- Morlion B (2011) Pharmacotherapy of low back pain: targeting nociceptive and neuropathic pain components. Curr Med Res Opin 27(1): 11-33.
- Chou R, Deyo R, Friedly J, Skelly A, Weimer M, et al. (2017) Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice guideline. Ann Intern Med 166(7): 480-492.
- Van der Gaag WH, Roelofs PDDM, Enthoven WTM, van Tulder MW, Koes BW, et al. (2020) non-steroidal anti-inflammatory drugs for acute low back pain. Cochrane Database of Systematic Reviews 4(4): CD013581.
- Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, et al. (2022) Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med 14(644): eabj9954.
- Astrup J, Gyntelberg F (2022) Tension-type headache and low back pain reconsidered. Front Neurol 13: 912348.
- Überall MA, Essner U, Müller-Schwefe GHH (2022) Efficacy and safety/tolerability of pridinol: a meta- analysis of double-blind, randomized, placebo-controlled trials in adult patients with muscle pain. Curr Med Res Opin 38(7): 1141-1151.
- Überall MA, Müller-Schwefe GHH, Horlemann J (2022) Efficacy and tolerability of the antispasmodic, pridinol, in patients with muscle-pain - results of PRIMEPAIN, a retrospective analysis of open- label real-world data provided by the German Pain e-Registry. Curr Med Res Opin 38(7): 1203-1217.
- (2023) Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale Versorgungs Leitlinie Nicht-spezifischer Kreuzschmerz – Langfassung, 2. Auflage. Version 1. 2017.
- Schjerning A-M, McGettigan P, Gislason G (2020) Cardiovascular effects, and safety of (non- aspirin) NSAIDs. Nat Rev Cardiol 17(9): 574-584.
- Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biology 231: 232-235.
- Fitzpatrick FA (2004) Cyclooxygenase enzymes: regulation and function. Curr Pharm Design 10(6): 577-588.
- Bonnesen K, Schmidt M (2021) Recategorization of non-aspirin nonsteroidal anti-inflammatory drugs according to clinical relevance: abandoning the traditional NSAID terminology. Canadian Journal of Cardiology 37(11): 1705-1707.
- Richter M, Donath F, Wedemeyer R-S, Warnke A, Horstmann A, et al. (2021) Pharmacokinetics of oral pridinol: results of a randomized, crossover bioequivalence trial in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 59(6): 471-477.
- Tune LE (2001) Anticholinergic effects of medication in elderly patients. J Clin Psychiatry 62: 11-14.
- Nishtala PS, Salahudeen MS, Hilmer SN (2016) Anticholinergics: Theoretical and clinical overview. Expert Opin Drug Saf 15(6): 753-768.
- Ramos H, Moreno L, Pérez-Tur J, Cháfer-Pericás C, García-Lluch G, et al. CRIDECO Anticholinergic Load Scale: An Updated Anticholinergic Burden Scale. Comparison with the ACB Scale in Spanish Individuals with Subjective Memory Complaints. J Pers Med 12(2): 207.
- (2020) German Pain Association. Manual for the German Pain Questionnaire (in German).
- (2023) The National Association of Statutory Health Insurance Physicians and the regional Associations of Statutory Health Insurance Physicians. Agreement on quality assurance measures according to § 135 Para. 2 SGB V for pain therapy care of patients with chronic pain (German: Vereinbarung von Qualitätssicherungsmaßnahmen nach § 135 Abs. 2 SGB V zur schmerztherapeutischen Versorgung chronisch schmerzkranker Patienten).
- Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70 (1): 41-55.
- Cohen J (1998) Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates. Fleiss J L. Measures of effect size for categorical data. In Cooper H & Hedges LV (Eds.), The handbook of research synthesis. 1994; Russell Sage Foundation pp. 245-260.
- Überall MA, Elling C, Eibl C, Müller-Schwefe GHH, Lefeber C, et al. (2022) Tapentadol prolonged release in patients with chronic low back pain: real-world data from the German Pain eRegistry. Pain Manag 12(2): 211-227.
- Tait RC, Chibnall JT, Krause S (1990) The pain disability index: psychometric properties. Pain 40(2): 171-182.
- Ware J Jr, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3): 220-233.
- Selim AJ, Rothendler JA, Qian SX, Bailey HM, Kazis LE, et al. (2022) The History and Applications of the Veterans RAND 12-Item Health Survey (VR-12). J Ambul Care Manage 45(3): 161-170.
- Ostelo RWJG, de Vet HCW (2005) Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 19(4): 593-607.
- María J Díaz-Arribas, Mónica Fernández-Serrano, Ana Royuela, Francisco M Kovacs, Tomás Gallego-Izquierdo, et al. (2017) Minimally clinically important difference in quality of life for patients with low back pain. Spine 42(24): 1908-1916.
- (2023) German Institute of Medical Documentation and Information (DIMDI). Anatomical-therapeutic- chemical classification with daily doses. Official version of the ATC index with DDD information for Germany in 2022.
- (2023) Regulation (EU) 2016/679 of the European Parliament and the Council on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation); van der Gaag WH, Roelofs PD, Enthoven WT, van Tulder MW, Koes BW. Non-steroidal anti- inflammatory drugs for acute low back pain. Cochrane Database Syst Rev 2020; (4): CD013582.
- Rathore FA, Afridi A (2021) Are non-steroidal anti-inflammatory drugs effective for acute low back pain? A Cochrane Review summary with commentary. Int J Rheum Dis 24(4): 611-614.
- Long B, Gottlieb M (2021) Nonsteroidal anti-inflammatory drugs for acute low-back pain. Acad Emerg Med 28(3): 372-374.
-
Michael A Überall*, Artur Schikowski, Johannes Horlemann and Gerhard HH Müller-Schwefe. Effectiveness Of the Antispasmodic Pridinol Vs. Nsaids in Patients with Acute (Low) Back Pain – Results of Providence, A Retrospective, Non-Interventional Propensity- Score Matched Dual Cohort Analysis of Depersonalized 4-Week Real-World Data Provided by The German Pain E-Registry. Arch Phar & Pharmacol Res. 4(2): 2024. APPR.MS.ID.000582.
-
Antispasmodic Pridinol, Acute (Low) Back Pain, E-Registry, Effectiveness, Nsaids, Score Matching, Propensity
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






